
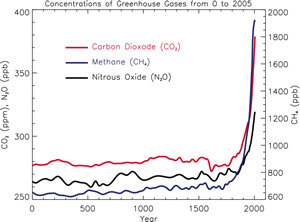
As one of the main greenhouse gases, carbon dioxide (CO2) plays a crucial role in regulating the Earth's temperature. Since the beginning of the 20th century, the rapid rise of CO2 in the atmosphere has been directly linked to observations of global warming at the Earth's surface. Atmospheric concentrations of carbon dioxide are currently 40% greater than pre-industrial concentrations and at their highest levels in human history. Their rise is directly due to to human activities such as the burning of fossil fuels (e.g. coal, petrol) and large scale changes in land use (e.g. deforestation).
It is known that the oceans act as a buffer against the accumulation of CO2 in the atmosphere. Since 1800, they have absorbed about one half of the CO2 emissions released by human activities. As a result the ocean is helping to moderate the impact of rising CO2 emissions on the Earth's climate. Understanding how the oceans' capacity to absorb CO2 may change in the future is fundamental to improving our ability to predict climate change. This task is made more difficult due to the influence of many factors.
The oceans absorption of CO2 is driven by the difference in concentration (measured as partial pressure, pCO2) between the atmosphere and the ocean (termed ΔpCO2). This difference is determined by a number of interactions
Although the capacity of the oceans to absorb CO2 is good news for tempering global warming in the short term, it also introduces new risks to marine life. This is because the more CO2 absorbed, the more acidic the oceans become. Increases in ocean acidity can be measured as a decrease in ocean pH, which is estimated to have declined by 0.1 units (about a 30% increase in acidity) since pre-industrial times.
Our understanding of the full impacts of changing pH on marine ecosystems is limited and requires further research. However, one of the better-known consequences is that organisms containing calcium carbonate shells (e.g. some corals, shellfish, zooplankton and phytoplankton) will find it harder to make calcium carbonate.
More information about CO2 and climate change is available in our references and further reading section.