Metadata Report for BODC Series Reference Number 2121664
Metadata Summary
Problem Reports
Data Access Policy
Narrative Documents
Project Information
Data Activity or Cruise Information
Fixed Station Information
BODC Quality Flags
SeaDataNet Quality Flags
Metadata Summary
Data Description |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Data Identifiers |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Time Co-ordinates(UT) |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Spatial Co-ordinates | |||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Parameters |
|||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||
Definition of BOTTFLAG | |||||||||||||||||||||||||||||||||||||
| BOTTFLAG | Definition |
|---|---|
| 0 | The sampling event occurred without any incident being reported to BODC. |
| 1 | The filter in an in-situ sampling pump physically ruptured during sample resulting in an unquantifiable loss of sampled material. |
| 2 | Analytical evidence (e.g. surface water salinity measured on a sample collected at depth) indicates that the water sample has been contaminated by water from depths other than the depths of sampling. |
| 3 | The feedback indicator on the deck unit reported that the bottle closure command had failed. General Oceanics deck units used on NERC vessels in the 80s and 90s were renowned for reporting misfires when the bottle had been closed. This flag is also suitable for when a trigger command is mistakenly sent to a bottle that has previously been fired. |
| 4 | During the sampling deployment the bottle was fired in an order other than incrementing rosette position. Indicative of the potential for errors in the assignment of bottle firing depth, especially with General Oceanics rosettes. |
| 5 | Water was reported to be escaping from the bottle as the rosette was being recovered. |
| 6 | The bottle seals were observed to be incorrectly seated and the bottle was only part full of water on recovery. |
| 7 | Either the bottle was found to contain no sample on recovery or there was no bottle fitted to the rosette position fired (but SBE35 record may exist). |
| 8 | There is reason to doubt the accuracy of the sampling depth associated with the sample. |
| 9 | The bottle air vent had not been closed prior to deployment giving rise to a risk of sample contamination through leakage. |
Definition of Rank |
|
|
Problem Reports
No Problem Report Found in the Database
Data Access Policy
Open Data
These data have no specific confidentiality restrictions for users. However, users must acknowledge data sources as it is not ethical to publish data without proper attribution. Any publication or other output resulting from usage of the data should include an acknowledgment.
If the Information Provider does not provide a specific attribution statement, or if you are using Information from several Information Providers and multiple attributions are not practical in your product or application, you may consider using the following:
"Contains public sector information licensed under the Open Government Licence v1.0."
Narrative Documents
ThermoFinnigan Neptune inductively coupled plasma mass spectrometer
A laboratory high mass resolution inductively coupled plasma mass spectrometer (ICP-MS) designed for elemental and isotopic analysis. The instrument is based on a multicollector platform and combines the features of high mass resolution, variable multicollection, zoom optics and multiple ion counting (MIC). It has a large mass dispersion (812 mm) using an ion optical magnification of two.
The Neptune ICP-MS includes eight moveable collector supports and one fixed center channel which are installed on the optical bench of the multicollector module. The center channel is equipped with a Faraday cup and, optionally, an ion counter with or without a retardation lens. The eight detector supports are driven by motors and can be precisely positioned along the focal plane according to need. Beneath each variable detector platform there is a position sensor located inside the vacuum chamber, allowing precise in situ monitoring of the detector position.
Each moveable support can carry a Faraday cup, a miniature Secondary Electron Multiplier (SEM) or a combination of the two. The large Faraday cups have been laser machined from solid carbon and each is connected to a current amplifier whose signal is digitised by a high linearity voltage to frequency converter. The amplifiers are mounted in a doubly shielded, evacuated and thermostated housing with a temperature stability of ± 0.01°C. Amplifier dynamic range is restricted to 15 volts in negative ion detection mode and 50 volts in positive ion detection mode. The instrument eliminates gain calibration biases by using the Virtual Amplifier concept whereby all Faraday cups involved in a certain measurement are sequentially connected to all amplifiers and it can detect up to 17 ion beams simultaneously.
The instrument includes refined ion optics which are almost coincident angular, energy image planes and large focal depth due to large ion optical magnification. It has three resolution settings and a wide detector slit for each of these. In high resolution settings the resolving power can go as high as m/Δm = 10.000. The m/Δm is derived from the peak slope of the rising edge measured at 5% and 95% relative peak height.
The instrument has been discontinued and ThermoFinnigan has been incorporated into Thermo Scientific (part of Thermo Fisher Scientific).
Niskin Bottle
The Niskin bottle is a device used by oceanographers to collect subsurface seawater samples. It is a plastic bottle with caps and rubber seals at each end and is deployed with the caps held open, allowing free-flushing of the bottle as it moves through the water column.
Standard Niskin
The standard version of the bottle includes a plastic-coated metal spring or elastic cord running through the interior of the bottle that joins the two caps, and the caps are held open against the spring by plastic lanyards. When the bottle reaches the desired depth the lanyards are released by a pressure-actuated switch, command signal or messenger weight and the caps are forced shut and sealed, trapping the seawater sample.
Lever Action Niskin
The Lever Action Niskin Bottle differs from the standard version, in that the caps are held open during deployment by externally mounted stainless steel springs rather than an internal spring or cord. Lever Action Niskins are recommended for applications where a completely clear sample chamber is critical or for use in deep cold water.
Clean Sampling
A modified version of the standard Niskin bottle has been developed for clean sampling. This is teflon-coated and uses a latex cord to close the caps rather than a metal spring. The clean version of the Levered Action Niskin bottle is also teflon-coated and uses epoxy covered springs in place of the stainless steel springs. These bottles are specifically designed to minimise metal contamination when sampling trace metals.
Deployment
Bottles may be deployed singly clamped to a wire or in groups of up to 48 on a rosette. Standard bottles and Lever Action bottles have a capacity between 1.7 and 30 L. Reversing thermometers may be attached to a spring-loaded disk that rotates through 180° on bottle closure.
Cruise DY021 total dissolved chromium concentration, chromium(III) concentration and isotopic composition
This dataset contains total dissolved chromium concentration, chromium(III) concentration and isotopic composition data from samples collected during RRS Discovery cruise DY021 (sampling period 08/03/2015 to 22/03/2015), as part of the UK Shelf Sea Biogeochemistry research programme
Originator's Protocol for Data Acquisition and Analysis
Sampling protocol for total dissolved Cr and δ53Cr: Ultra-clean CTD bottles containing seawater were transferred to class 100 clean laboratory aboard RRS Discovery and seawater was filtered through Sartorius Sartobran 0.45 µm capsule filters. Acid-washed 1 L LDPE bottles were rinsed 3 times with sample before the final seawater sample was collected (5 x 1 L per sample). These were stored double-bagged and tranferred to the National Oceanography Centre (Southampton, UK) on 26th March 2015, where they were acidified using 2 mL L-1 sub-boiled hydrochloric acid.Samples were stored for ~10 months before analysis.
Sampling protocol for Cr(III): Ultra-clean CTD bottles containing seawater were transferred to class 100 clean laboratory aboard RRS Discovery and seawater was filtered through Sartorius Sartobran 0.45 µm capsule filters. Acid-washed 500 mL LDPE bottles were rinsed 3 times with sample before the final seawater sample was collected (1 x 500 mL per sample). The samples were treated with a53Cr isotopic spike and Fe(III) hydroxide solution to capture Cr(III) (Cranston and Murray, 1978) within ~1 hour of collection. They were double-bagged and transported back to NOCS and stored for ~5 months before analysis.
Analytical protocol for total dissolved Cr and δ53Cr: Samples were treated with an isotopic double spike (50Cr-54Cr). Approx. 24 hours later, sample pH was adjusted to 8-9 using ammonia, then Cr was removed from the seawater matrix using a Fe(II) co-precipitation technique (Bonnand et al. 2013; Cranston and Murray, 1978). The resulting Cr-Fe precipitates were filtered using vacuum filtration through pre-cleaned Millipore Omnipore filters (1 µm) and purified using a two stage ion chromatography procedure. Samples were then treated with hydrogen peroxide to oxidise any remaining organic material and analysed in 3% nitric acid using the Thermo Fisher Neptune MC-ICP-MS in medium resolution at NOCS. Blank 3% nitric acid solutions were measured between samples and resulting voltages subtracted from the sample voltages. Values of δ53Cr were calculated using a Newton-Raphson deconvolution calculation and expressed relative to the NBS979 standard (δ53Cr = 0‰ see Bonnand et al. 2013). NBS979 Cr standards were also repeatedly analysed during each anaytical session and initial sample δ53Cr values were corrected for instrumental drift using these. Finally, a correction to account for the procedural blank (derived from the Fe(II) hydroxide precipitate) was applied. Total dissolved Cr values were calculated by applying isotope dilution calculations to MC-ICP-MS data (e.g. Ohata et al. 1998) and a blank subtraction (using the concentration of Cr in the Fe(II) solution) was applied.
Analytical protocol for Cr(III): Cr-Fe precipitates resulting from on-board sample treatment were filtered using vacuum filtration through pre-cleaned Millipore Omnipore filters (11 µm) and purified using a two stage ion chromatography procedure (Bonnand et al. 2013). Samples were then treated with hydrogen peroxide to oxidise any remaining organic material and analysed in 3% nitric acid using the Element 2 ICP-MS. Cr(III) values were calculated by applying isotope dilution calculations (e.g. Ohata et al. 1998) and a blank subtraction (using the total procedural blank for an equivalent volume of Milli-Q water) was applied.
References Cited
Bonnand, P., James, R.H., Parkinson, I.J., Connelly, D.P. and Fairchild, I.J., 2013. The chromium isotopic composition of seawater and marine carbonates. Earth. Planet. Sci. Lett. 382, 10-20.
Cranston, R.E., and Murray, J.W. 1978. The determination of chromium species in natural waters. Anal. Chem. Act., 99, 275-282.
Ohata, M., Ichinose, T. Furuta, N., Shinohara A. and Chiba M. 1998. Isotope dilution analysis of Se in human blood serum by using high power nitrogen microwave-induced plasma mass spectrometry coupled with a hydride generation technique", Anal. Chem., 70(13), 2726-2730
BODC Data Processing Procedures
Data were provided in an Excel spreadsheet and archived at BODC. The file contained the water sample data from an UltraClean CTD (UCCTD) bottles. Data received were loaded into the BODC database using established BODC data banking procedures. The data were loaded into BODC's database without any changes. The originator variables were mapped to appropriate BODC parameter codes as follows:
The originator variables were mapped to appropriate BODC parameter codes as follows:
| Originator's Parameter | Unit | Description | BODC Parameter Code | BODC Unit | Comment |
|---|---|---|---|---|---|
| δ53Cr | [‰] | Enrichment with respect to 53Cr/52Cr in NBS979 of chromium-53 {53Cr} {δ53Cr} in the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration and inductively-coupled plasma mass spectrometry | EN53CR01 | Parts per thousand | |
| δ53Cr analytical 2 SD | [‰] | Enrichment with respect to 53Cr/52Cr in NBS979 standard deviation of chromium-53 {53Cr} {δ53Cr} in the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration and inductively-coupled plasma mass spectrometry | EN53CRSD | Parts per thousand | Original values diveded by 2 |
| Total Cr | [nmol/kg] | Concentration of chromium {Cr CAS 7440-47-3} per unit mass of the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration, acidification and inductively-coupled plasma mass spectrometry | CRXXX001 | Nanomoles per kilogram | |
| Total Cr analytical 2 SD | [nmol/kg] | Concentration standard deviation of chromium {Cr CAS 7440-47-3} per unit mass of the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration, acidification and inductively-coupled plasma mass spectrometry | CRXSD001 | Nanomoles per kilogram | Original values diveded by 2 |
| Cr(III) | [nmol/kg] | Concentration of chromium, trivalent {Cr3+ CAS 16065-83-1} per unit mass of the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration, precipitation, acidification and inductively-coupled plasma mass spectrometry | CR3XX001 | Nanomoles per kilogram | |
| Cr(III) analytical 2 SD | [nmol/kg] | Concentration standard deviation of chromium, trivalent {Cr3+ CAS 16065-83-1} per unit mass of the water body [dissolved plus reactive particulate < 0.4/0.45 µm phase] by filtration, precipitation, acidification and inductively-coupled plasma mass spectrometry | CR3SD001 | Nanomoles per kilogram | Original values diveded by 2 |
Project Information
Shelf Sea Biogeochemistry (SSB) Programme Work Package 3: Supply of iron from shelf sediments to the ocean
Work Package 3 is a £0.78 million component of the Natural Environment Research Council (NERC) Shelf Sea Biogeochemistry (SSB) research programme, running from 2013 to 2017. It is jointly funded by NERC and the Department for Environment, Food and Rural Affairs (DEFRA). The goal of this project is to quantify iron (Fe) fluxes from the north-west European shelf seas to the adjacent North Atlantic Ocean.
Background
Low iron (Fe) concentrations control productivity, phytoplankton community structure and carbon cycling in 25 % of the open ocean. Iron concentrations are tightly coupled to Fe supply, and Fe fluxes from shelf seas to the open ocean are poorly constrained, although estimates indicate they could be 2-10 times higher than atmospheric inputs and thus potentially a major contributor to the oceanic Fe cycle.
The goal of Work Package 3 will be realised during cruises in the Celtic Sea as part of the Shelf Sea Biogeochemistry Research Programme (SSB), which will provide the physical and chemical context for our study. The data collection will utilise trace metal clean sampling techniques, with associated physical diffusion and advection measurements, to determine the supply of dissolved, colloidal and particulate forms of Fe from sediments and their subsequent fate in shelf sea waters and during export to the North Atlantic Ocean. Data analyses will use Fe isotopes, physical-chemical Fe species characterisation and geochemical tracers to quantify the Fe supply, attenuation and export processes.
Further details are available on the SSB website.
Participants
6 different organisations are directly involved in research for SSB Work Package 3. These institutions are
- Centre for Environment, Fisheries and Aquaculture Science (Cefas)
- National Oceanography Centre (NOC)
- Plymouth University
- University of Edinburgh
- University of Oxford
- University of Southampton
Objectives
This Work Package aims to:
-
Study the processes whereby iron is released from shelf sediments into overlying waters, and how these mechanisms can be influenced by organic matter from decaying plant material
-
Provide new information on processes influencing release of iron to shelf waters to allow improved modelling of the size of this source, and of the key processes involved. For example, organic carbon inputs are expected to be associated with iron releases from the sediments, and using models will help extrapolate into the future and how the system will respond to climate change.
-
Link the processes impacting iron in shelf waters with physical models and radionuclide estimates of the movement of water off shelf to give new estimates of the size of this source to the ocean. This information will have implications for shelf break and ocean productivity.
-
Help interpret existing collected data (sediment profile images) on organic carbon within sediments and the status of the seabed through specific studies of sediment iron geochemistry. Specific studies of sediment iron geochemistry will help better interpret presently collected data (sediment profile images) on organic carbon within sediments and status of the seabed.
Fieldwork and data collection
Data for Work Package 3 will be gathered on all process cruises. These are listed in the table below. The study area is the marine shelf (and shelf-edge) of the Celtic Sea. Work will be carried out on board the NERC research vessels RRS Discovery and RRS James Cook.
| Cruise identifier | Research ship | Cruise dates | Work packages |
|---|---|---|---|
| DY008 | RRS Discovery | March 2014 | WP 2 and WP 3 |
| JC105 | RRS James Cook | June 2014 | WP 1, WP 2 and WP 3 |
| DY026 | RRS Discovery | August 2014 | WP1, WP 2 and WP 3 |
| DY018 | RRS Discovery | November - December 2014 | WP 1 and WP 3 |
| DY021 (also known as DY008b) | RRS Discovery | March 2015 | WP 2 and WP 3 |
| DY029 | RRS Discovery | April 2015 | WP 1 and WP 3 |
| DY030 | RRS Discovery | May 2015 | WP 2 and WP 3 |
| DY033 | RRS Discovery | July 2015 | WP 1 and WP 3 |
| DY034 | RRS Discovery | August 2015 | WP 2 and WP 3 |
Activities will include iron and radium measurements, glider work, coring, CTDs, stand alone pumps (SAPS), incubations with sediments and sea water.
Data Activity or Cruise Information
Data Activity
| Start Date (yyyy-mm-dd) | 2015-03-08 |
| End Date (yyyy-mm-dd) | 2015-03-08 |
| Organization Undertaking Activity | Plymouth Marine Laboratory |
| Country of Organization | United Kingdom |
| Originator's Data Activity Identifier | DY021_UCCTD_118 |
| Platform Category | lowered unmanned submersible |
BODC Sample Metadata Report for DY021_UCCTD_118
| Sample reference number | Nominal collection volume(l) | Bottle rosette position | Bottle firing sequence number | Minimum pressure sampled (dbar) | Maximum pressure sampled (dbar) | Depth of sampling point (m) | Bottle type | Sample quality flag | Bottle reference | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 985691 | 10.00 | 1 | 61.70 | 62.70 | 60.90 | Teflon-coated Niskin bottle | No problem reported | |||
| 985694 | 10.00 | 2 | 61.70 | 62.70 | 60.90 | Teflon-coated Niskin bottle | No problem reported | |||
| 985697 | 10.00 | 3 | 61.90 | 62.90 | 61.10 | Teflon-coated Niskin bottle | No problem reported | |||
| 985700 | 10.00 | 4 | 61.50 | 62.50 | 60.70 | Teflon-coated Niskin bottle | No problem reported | |||
| 985703 | 10.00 | 5 | 62.10 | 63.10 | 61.30 | Teflon-coated Niskin bottle | No problem reported | |||
| 985706 | 10.00 | 6 | 62.00 | 63.00 | 61.20 | Teflon-coated Niskin bottle | No problem reported | |||
| 985709 | 10.00 | 7 | 61.10 | 62.10 | 60.30 | Teflon-coated Niskin bottle | No problem reported | |||
| 985712 | 10.00 | 8 | 61.90 | 62.90 | 61.10 | Teflon-coated Niskin bottle | No problem reported | |||
| 985715 | 10.00 | 9 | 61.20 | 62.20 | 60.40 | Teflon-coated Niskin bottle | No problem reported | |||
| 985718 | 10.00 | 10 | 96.80 | 97.80 | 95.70 | Teflon-coated Niskin bottle | No problem reported | |||
| 985721 | 10.00 | 11 | 96.50 | 97.50 | 95.40 | Teflon-coated Niskin bottle | No problem reported | |||
| 985724 | 10.00 | 14 | 88.20 | 89.20 | 87.10 | Teflon-coated Niskin bottle | No problem reported | |||
| 985727 | 10.00 | 15 | 87.30 | 88.30 | 86.20 | Teflon-coated Niskin bottle | No problem reported | |||
| 985730 | 10.00 | 16 | 75.80 | 76.80 | 74.80 | Teflon-coated Niskin bottle | No problem reported | |||
| 985733 | 10.00 | 17 | 77.30 | 78.30 | 76.30 | Teflon-coated Niskin bottle | No problem reported | |||
| 985736 | 10.00 | 18 | 61.70 | 62.70 | 60.90 | Teflon-coated Niskin bottle | No problem reported | |||
| 985739 | 10.00 | 19 | 61.50 | 62.50 | 60.70 | Teflon-coated Niskin bottle | No problem reported | |||
| 985742 | 10.00 | 20 | 42.10 | 43.10 | 41.40 | Teflon-coated Niskin bottle | No problem reported | |||
| 985745 | 10.00 | 21 | 41.40 | 42.40 | 40.80 | Teflon-coated Niskin bottle | No problem reported | |||
| 985748 | 10.00 | 22 | 27.20 | 28.20 | 26.70 | Teflon-coated Niskin bottle | No problem reported | |||
| 985751 | 10.00 | 23 | 26.90 | 27.90 | 26.40 | Teflon-coated Niskin bottle | No problem reported | |||
| 1310858 | 13 | 93.00 | Teflon-coated Niskin bottle | No problem reported |
Please note:the supplied parameters may not have been sampled from all the bottle firings described in the table above. Cross-match the Sample Reference Number above against the SAMPRFNM value in the data file to identify the relevant metadata.
Related Data Activity activities are detailed in Appendix 1
Cruise
| Cruise Name | DY021 |
| Departure Date | 2015-03-01 |
| Arrival Date | 2015-03-26 |
| Principal Scientist(s) | E Malcolm S Woodward (Plymouth Marine Laboratory) |
| Ship | RRS Discovery |
Complete Cruise Metadata Report is available here
Fixed Station Information
Fixed Station Information
| Station Name | Shelf Seas Biogeochemistry Fixed Station Benthic G |
| Category | Offshore area |
| Latitude | 51° 4.40' N |
| Longitude | 6° 34.85' W |
| Water depth below MSL | 103.0 m |
Shelf Seas Biogeochemistry Fixed Station Benthic G
This station is one of four benthic sites sampled on the Celtic Sea shelf as part of work package II of the Shelf Seas Biogeochemistry project. The station has a mean water depth 109 m at the following co-ordinates:
| Box Corner | Latitude | Longitude |
|---|---|---|
| North-west corner | 51.0764° | -6.5848° |
| South-east corner | 51.0702° | -6.5770° |
The position of this station relative to the other Shelf Seas Biogeochemistry sites can be seen from the figure below.
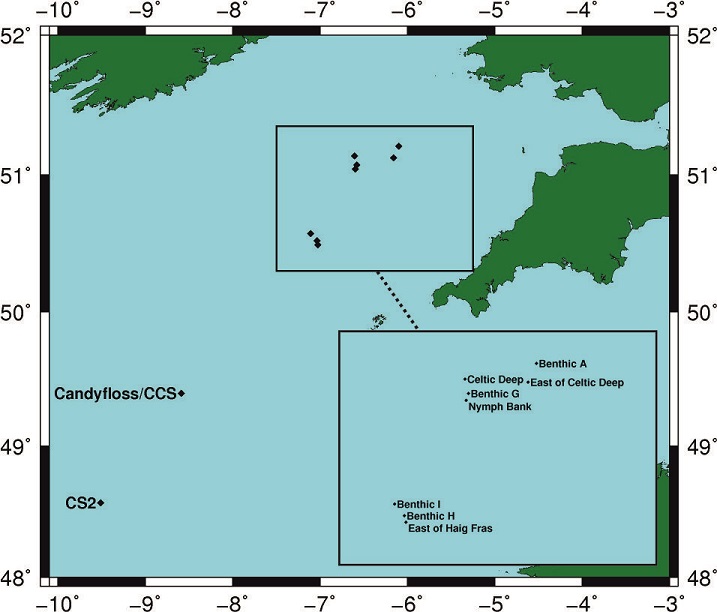
Sampling History
| DY008 | |
|---|---|
| CTD casts | 2 |
| Box cores | 65 |
| SPI camera | 19 |
| Stand Alone Pump Systems (SAPS) | 1 |
| Benthic flume | 2 |
| Autosub6000 | 2 |
| Glider deployments | 1 |
Mooring deployments
| Latitude | Longitude | Water depth (m) | Moored instrument | Deployment date | Recovery date | Deployment cruise | Recovery cruise |
|---|---|---|---|---|---|---|---|
| 51.0749° | -6.5848° | 100 | NOC-L benthic lander | 31-03-2014 12:54 UTC | 07-04-2014 09:09 UTC | DY008 | DY008 |
Related Fixed Station activities are detailed in Appendix 2
BODC Quality Control Flags
The following single character qualifying flags may be associated with one or more individual parameters with a data cycle:
| Flag | Description |
|---|---|
| Blank | Unqualified |
| < | Below detection limit |
| > | In excess of quoted value |
| A | Taxonomic flag for affinis (aff.) |
| B | Beginning of CTD Down/Up Cast |
| C | Taxonomic flag for confer (cf.) |
| D | Thermometric depth |
| E | End of CTD Down/Up Cast |
| G | Non-taxonomic biological characteristic uncertainty |
| H | Extrapolated value |
| I | Taxonomic flag for single species (sp.) |
| K | Improbable value - unknown quality control source |
| L | Improbable value - originator's quality control |
| M | Improbable value - BODC quality control |
| N | Null value |
| O | Improbable value - user quality control |
| P | Trace/calm |
| Q | Indeterminate |
| R | Replacement value |
| S | Estimated value |
| T | Interpolated value |
| U | Uncalibrated |
| W | Control value |
| X | Excessive difference |
SeaDataNet Quality Control Flags
The following single character qualifying flags may be associated with one or more individual parameters with a data cycle:
| Flag | Description |
|---|---|
| 0 | no quality control |
| 1 | good value |
| 2 | probably good value |
| 3 | probably bad value |
| 4 | bad value |
| 5 | changed value |
| 6 | value below detection |
| 7 | value in excess |
| 8 | interpolated value |
| 9 | missing value |
| A | value phenomenon uncertain |
| B | nominal value |
| Q | value below limit of quantification |
Appendix 1: DY021_UCCTD_118
Related series for this Data Activity are presented in the table below. Further information can be found by following the appropriate links.
If you are interested in these series, please be aware we offer a multiple file download service. Should your credentials be insufficient for automatic download, the service also offers a referral to our Enquiries Officer who may be able to negotiate access.
| Series Identifier | Data Category | Start date/time | Start position | Cruise |
|---|---|---|---|---|
| 2119660 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
| 2127395 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
| 2134331 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
Appendix 2: Shelf Seas Biogeochemistry Fixed Station Benthic G
Related series for this Fixed Station are presented in the table below. Further information can be found by following the appropriate links.
If you are interested in these series, please be aware we offer a multiple file download service. Should your credentials be insufficient for automatic download, the service also offers a referral to our Enquiries Officer who may be able to negotiate access.
| Series Identifier | Data Category | Start date/time | Start position | Cruise |
|---|---|---|---|---|
| 1371604 | CTD or STD cast | 2014-04-02 17:42:00 | 51.07287 N, 6.58117 W | RRS Discovery DY008 |
| 1336735 | Water sample data | 2014-04-02 17:43:00 | 51.07285 N, 6.58116 W | RRS Discovery DY008 |
| 1371616 | CTD or STD cast | 2014-04-04 11:47:00 | 51.0726 N, 6.58027 W | RRS Discovery DY008 |
| 2117561 | Water sample data | 2014-04-04 11:50:30 | 51.0726 N, 6.58026 W | RRS Discovery DY008 |
| 2119113 | Water sample data | 2014-04-04 11:50:30 | 51.0726 N, 6.58026 W | RRS Discovery DY008 |
| 1336747 | Water sample data | 2014-04-04 11:51:00 | 51.0726 N, 6.58026 W | RRS Discovery DY008 |
| 2119660 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
| 2127395 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
| 2134331 | Water sample data | 2015-03-08 07:42:30 | 51.07249 N, 6.58106 W | RRS Discovery DY021 |
| 2118042 | Water sample data | 2015-03-08 10:50:00 | 51.07241 N, 6.58103 W | RRS Discovery DY021 |
| 2127230 | Water sample data | 2015-03-08 10:50:00 | 51.07241 N, 6.58103 W | RRS Discovery DY021 |
| 2135844 | Water sample data | 2015-03-08 10:50:00 | 51.07241 N, 6.58103 W | RRS Discovery DY021 |
| 1624669 | CTD or STD cast | 2015-05-06 13:57:00 | 51.07416 N, 6.58435 W | RRS Discovery DY030 |
| 2132373 | Water sample data | 2015-05-06 14:09:00 | 51.07491 N, 6.58435 W | RRS Discovery DY030 |
| 2137734 | Water sample data | 2015-05-06 14:09:00 | 51.07491 N, 6.58435 W | RRS Discovery DY030 |
| 1624670 | CTD or STD cast | 2015-05-06 15:33:00 | 51.07491 N, 6.58437 W | RRS Discovery DY030 |
| 1624589 | CTD or STD cast | 2015-05-06 17:03:00 | 51.07492 N, 6.5843 W | RRS Discovery DY030 |
| 2123370 | Water sample data | 2015-05-06 17:17:00 | 51.07492 N, 6.58436 W | RRS Discovery DY030 |
| 1624682 | CTD or STD cast | 2015-05-08 08:30:00 | 51.14136 N, 6.57319 W | RRS Discovery DY030 |
| 1624694 | CTD or STD cast | 2015-05-08 14:47:00 | 51.07506 N, 6.58458 W | RRS Discovery DY030 |
| 2132397 | Water sample data | 2015-05-08 14:57:30 | 51.07507 N, 6.58459 W | RRS Discovery DY030 |
| 2137746 | Water sample data | 2015-05-08 14:57:30 | 51.07507 N, 6.58459 W | RRS Discovery DY030 |
| 1624786 | CTD or STD cast | 2015-05-13 08:51:00 | 51.07433 N, 6.58489 W | RRS Discovery DY030 |
| 1624798 | CTD or STD cast | 2015-05-13 14:40:00 | 51.07452 N, 6.58398 W | RRS Discovery DY030 |
| 1721279 | CTD or STD cast | 2015-08-08 15:22:00 | 51.06715 N, 6.58203 W | RRS Discovery DY034 |
| 2122262 | Water sample data | 2015-08-08 15:39:30 | 51.06715 N, 6.58202 W | RRS Discovery DY034 |
| 1721292 | CTD or STD cast | 2015-08-10 11:14:00 | 51.07085 N, 6.57735 W | RRS Discovery DY034 |
| 1721556 | CTD or STD cast | 2015-08-11 15:32:00 | 51.07243 N, 6.58127 W | RRS Discovery DY034 |
| 2119715 | Water sample data | 2015-08-11 15:45:30 | 51.07243 N, 6.58124 W | RRS Discovery DY034 |
| 1721311 | CTD or STD cast | 2015-08-11 16:31:00 | 51.07242 N, 6.58113 W | RRS Discovery DY034 |
| 1721323 | CTD or STD cast | 2015-08-11 17:36:00 | 51.0724 N, 6.58103 W | RRS Discovery DY034 |
| 1721359 | CTD or STD cast | 2015-08-13 15:50:00 | 51.07262 N, 6.58162 W | RRS Discovery DY034 |
| 1721624 | CTD or STD cast | 2015-08-29 06:16:00 | 51.07275 N, 6.58083 W | RRS Discovery DY034 |


